PROJECT DETAILS
Optimal lung deposition of medication depends on the inbuilt resistance of the inhaler and the respiratory flow generated by the patient. Our client is a multinational conglomerate company based in London. Our collaboration has enabled them to analyze flow resistance in the dry powder inhaler for a range of inspiratory flow generated by patients from mild to severe chronic obstructive pulmonary disease. We employed precise CFD analysis practices to accurately capture the boundary layer and adopted meticulous verification steps to justify CFD analysis results. We delivered technically convincing CFD analysis results close to experimental values.

We Got More
Go through All Our Case Studies
CFD analysis of closed-loop anesthesia delivery system (CLADS)
Closed loop anesthesia delivery systems are specifically engineered to automate and enhance the administration of anesthesia throughout surgical procedures. By constantly monitoring crucial indicators like oxygen levels and breathing patterns, these systems intelligently adapt the anesthesia dosage to ensure the patient receives the precise amount required at any given moment. This not only enhances the overall efficiency of the procedure but also significantly improves its safety. Read More
Thermal analysis of high voltage X-ray tank assembly
The client is a global medical device OEM, one of the fastest growing in this segment. The client has approached us with the existing design of the high-voltage X ray tank unit. The client is interested in understanding the heat transfer phenomena for the complete system, which enables him to make conscious engineering decisions to enhance the performance of the device. Our CFD model is validated with the experimental results. CFD model is finetuned in all possible aspects, and we ensured that it represents the actual physical phenomena. All the heat transfer paths are analyzed, and the heat balance sheet has enabled our client to take well-informed engineering designs. Read MoreComputational fluid dynamics analysis of neonatal body temperature controller unit
The Neonatal body temperature controller, an initiative by the Government of India, aims to protect newborn babies from Hypoxic ischemic encephalopathy. Our client is working with the Government of India and the Government of Karnataka state to develop this technology for usage in Primary Health care Centers in rural and remote areas. Read More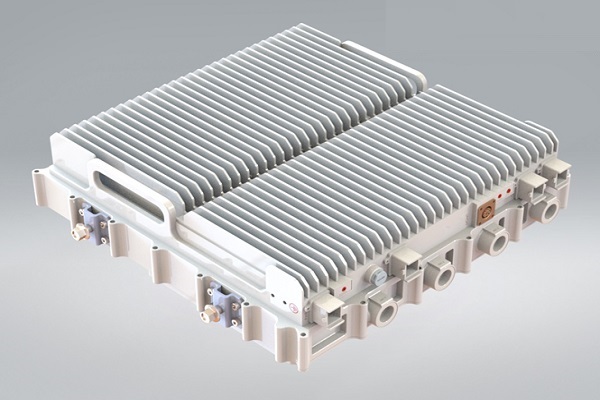
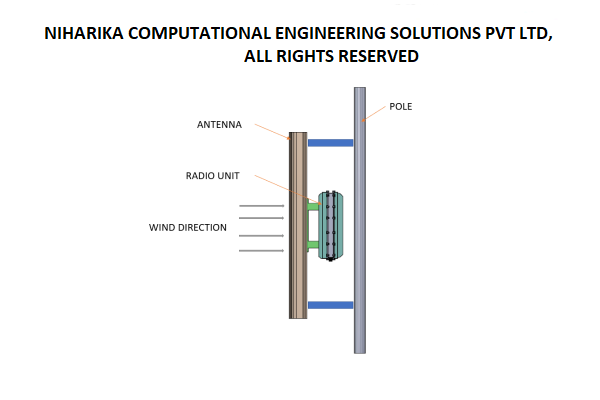
CFD analysis of Multi Band Remote Radio Unit
The advent of 5G technology has brought about unprecedented advancements in wireless communications, enabling faster speeds, lower latency, and increased capacity. These advancements are associated with higher power consumption and increased heat generation in the 5G Remote Radio units.Increasing data rates and network densification require radio units to process larger volumes of data, leading to higher power consumption and heat generation. Environmental factors such as ambient temperature, humidity, and exposure to direct sunlight also impact the thermal aspects of the radio units. Read More